20% Off store-wide. The promotion ends 25 Thursday at midnight. T&Cs apply. This offer is not redeemable for cash or gift cards, nor is it valid toward previous purchases. Offer may not be combined with any other coupons, discounts, offers, or promotions.
Hemp Farm®, Be The Seed Of Change™
The Best Hemp Products | Sustainable Nutrition
Plant-Based Protein, Whole foods & Superfoods, Supplements & Skincare
Hemp New Zealand™ Ltd is the largest hemp grower in New Zealand. Our well-known brands Hemp Farm® (Foods & Supplements) and Promise® (Natural Skincare) are *vegan-certified, *organic, nutritious, spray-free, and provide countless health and environmental benefits! “We are passionate about caring for YOU while protecting our precious WORLD”. We have been growing industrial hemp to supply industries with sustainable solutions since 2008. Be the seed of change™!
Read our story.
*Our healing balm contains manuka oil and beeswax. Hemp Farm® organic hemp seed oil is only available in 250ml.
Sustainable Nutrition
The Perfect Balance
Join Our Community & Save
Low Shipping Rates
* New Zealand Only & below 5kg
Our Best Sellers
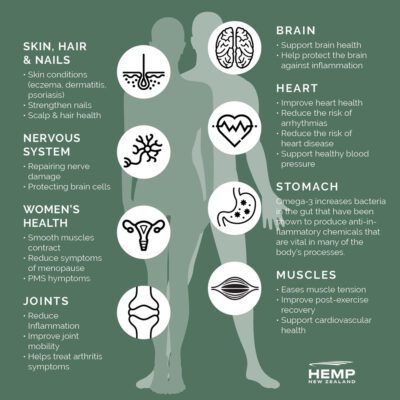
Supports healthy joints, skin, heart and brain.
Natural skincare - Suitable for the whole family!
Maintaining healthy skin is essential for your overall wellbeing. Our skin is the largest organ in our body, and it deserves the best care possible. Our natural products are designed to nourish and protect your skin, leaving it looking and feeling its best.